KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Anode-free lithium battery enabled by electron-deficient current collector
Anode-free lithium battery enabled by electron-deficient current collector
An anode-free lithium (Li) battery, free of Li metal when initially assembled, represents an exceptional advance for Li-based anodes. Here, an anode-free Li battery has been developed by tuning the electronic structure of the current collector. The electron-deficient current collector achieves high reversibility of Li plating/stripping, and improves the cycling stability of the anode-free cell under practical conditions.
Article | Spring 2022
To achieve high energy density lithium (Li) metal batteries, a low-Li source in the anode and a low electrolyte amount are considered requisite conditions. From this aspect, an anode-free Li metal battery, free of Li metal when initially assembled, represents an exceptional advance in Li-metal cell technology. In the anode-free battery, Li is electrodeposited on the anode current collector during the first charging process. Because Li foil is not used during cell assembly, there are great advantages in terms of energy density and cell manufacturing.
However, a long-cycling anode-free Li battery is hindered by continuous capacity degradation due to the lack of an excess Li source. Unlike a typical Li-metal battery, in which an additional Li source is inserted into the negative electrode in the form of Li foil, the irreversible capacity of the Li negative electrode cannot be compensated for in the anode-free configuration. In addition, a larger degree of electrolyte decomposition occurs at the current collector–electrolyte interface, which also causes losses of Li source and electrolyte.
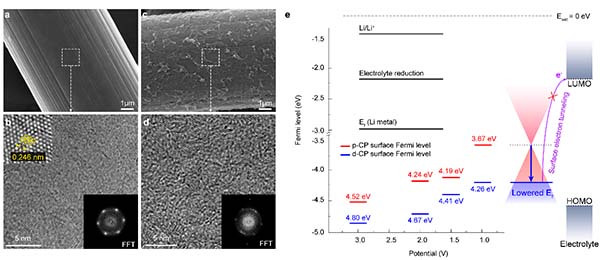
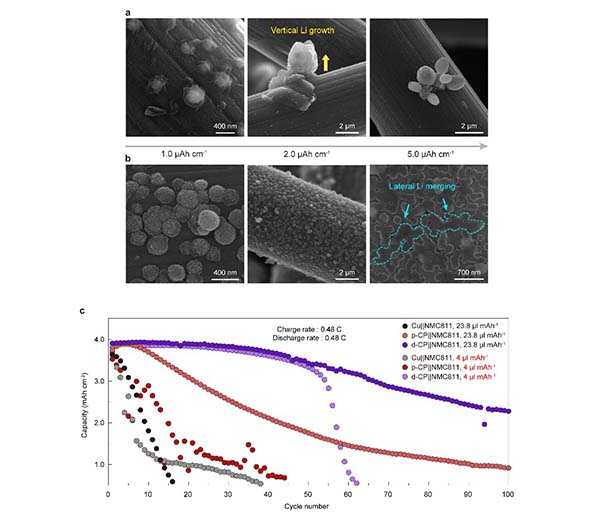
Prof. Hee-Tak Kim’s group in the Department of Chemical and Biomolecular Engineering at KAIST proposed the concept of tuning the electronic structure of the current collector to enable stable operation of anode-free Li batteries. The research group found that the defective carbon-induced electron-deficient current collector suppresses decomposition of the electrolyte on the current collector and imparts strong binding with Li metal, resulting in dendrite-free Li morphology.
The group revealed that, rather than forming a non-uniform mosaic structure, the electron-deficiency of the presented current collector facilitated the formation of a thin and uniform solid-electrolyte interphase (SEI) structure, which concurrently solves the challenges of anode-free Li batteries of continuous electrolyte consumption and uneven Li nucleation on current collector. Furthermore, the improved electron transfer between the electron-rich Li metal (low electronegativity) and electron-deficient current collector was the reason for the strong interaction between Li and the developed current collector, thereby realizing lateral Li growth without vertical and dendritic Li growth. As a result, the electron-deficient current collector enhanced the cycle life of the anode-free Li battery while minimizing electrolyte consumption and delaying Li exhaustion under practical conditions. This research was published in Nature Communications under the title “An electron-deficient carbon current collector for anode-free Li-metal batteries” (DOI:10.1038/s41467-021-25848-1).
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more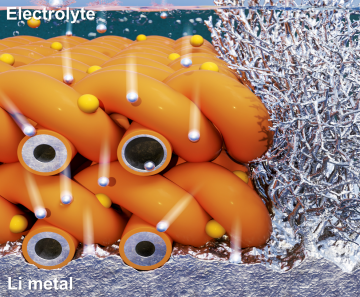
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more