KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Extending the lifespan of next-generation lithium metal batteries with water
KAIST developed eco-friendly hollow nanofiber protective layers for lithium-metal batteries, enhancing lifespan by 750%. Made from guar gum via a water-based process, these layers stabilize interfaces, control lithium growth, and naturally decompose, advancing sustainable battery technology.
A research team led by Professor Il-Doo Kim from the Department of Materials Science and Engineering at KAIST, in collaboration with Professor Jiyoung Lee from Ajou University, has successfully developed a novel eco-friendly method to produce hollow nanofibers as a protective layer for lithium metal. This innovation layer stabilizes lithium growth and significantly extends the lifespan of next-generation lithium-metal batteries.
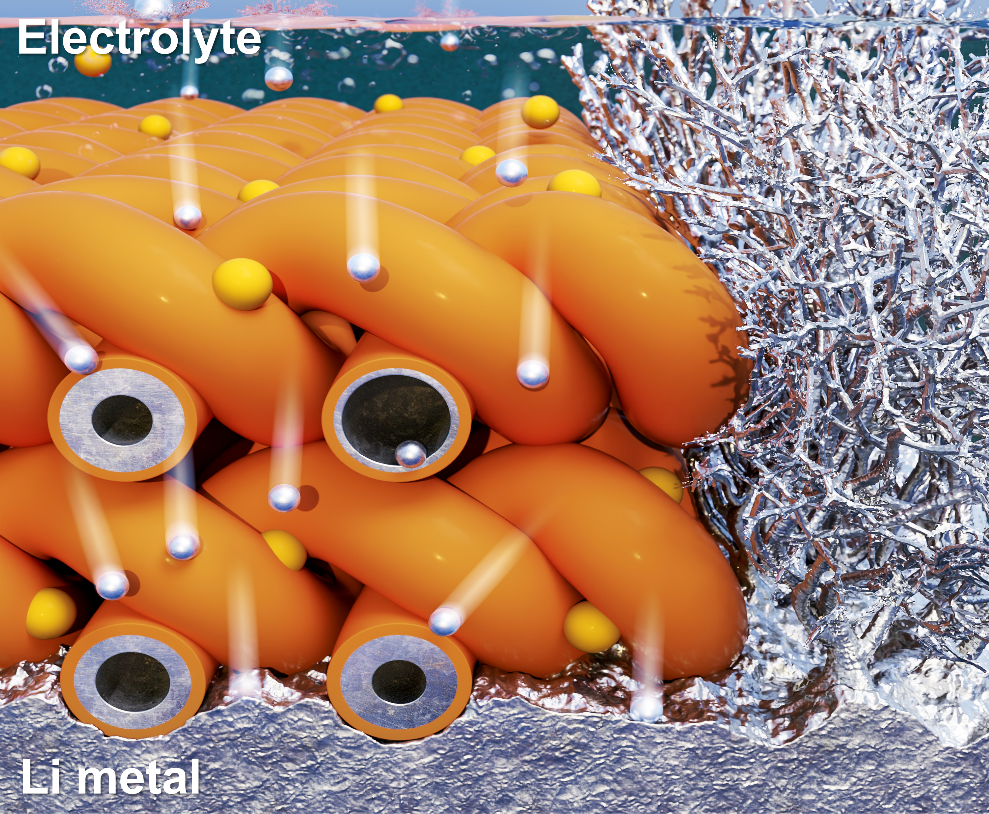
Conventional protective layers for lithium metal involve creating an artificial interface between the lithium metal and the electrolyte. However, these methods often rely on costly materials and hazardous processes, and they have limitations in significantly improving the lifespan of lithium-metal anodes. To address these limitations, the research team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth both physically and chemically.
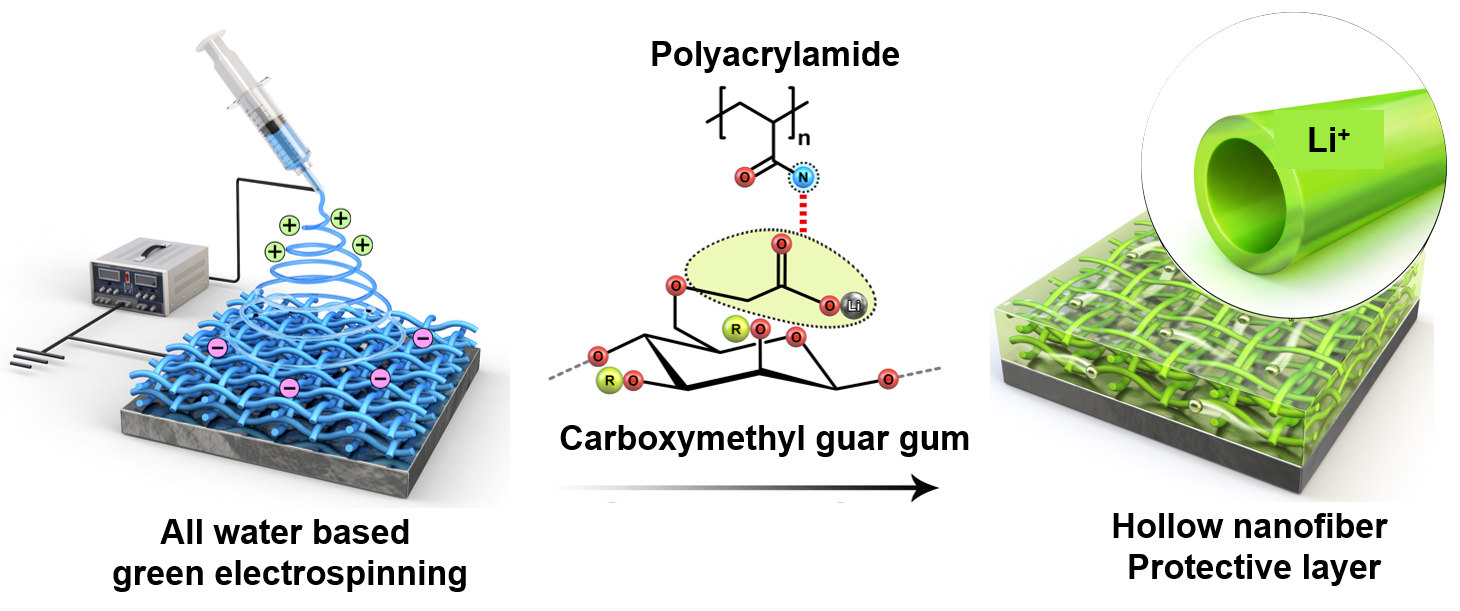
The newly developed protective layer is composed of guar gum, a plant-derived, eco-friendly polymer. The layer is manufactured using a green electrospinning process that requires only water, eliminating the need for harmful chemicals (Figure 1). This protective layer effectively regulates reversible chemical reactions between the electrolyte and lithium ions. Additionally, the hollow interior of the nanofibers prevents the random accumulation of lithium ions on the metal surface, achieving simultaneous stabilization of the interface between the lithium metal and the electrolyte.
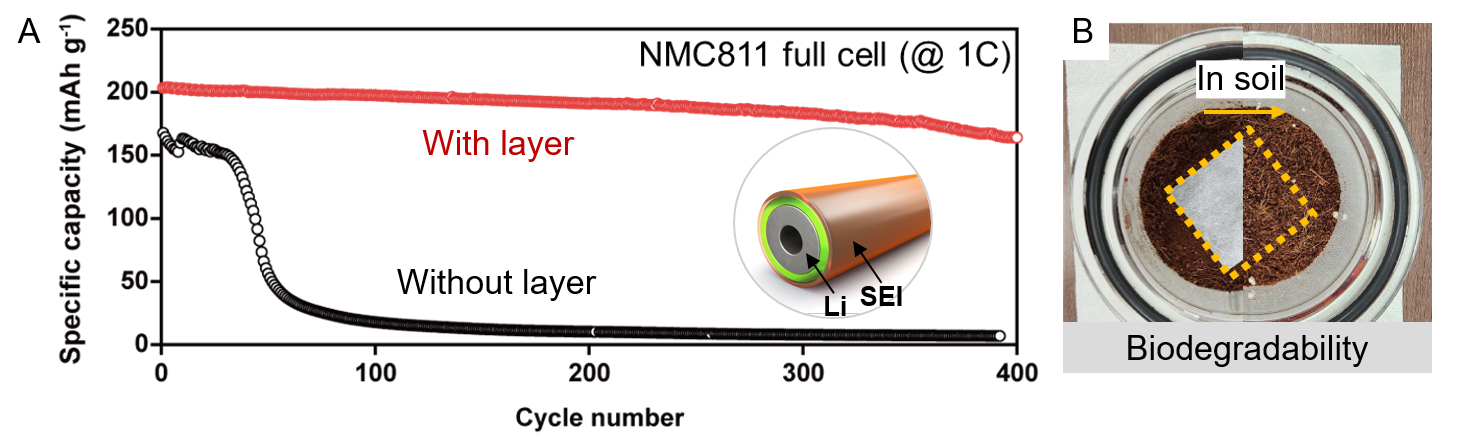
Tests revealed that lithium-metal anodes equipped with this protective layer exhibited a 750% improvement in lifespan compared to conventional lithium-metal anodes (Figure 2A). Even after 300 cycles of charge and discharge, the anodes retained approximately 93.3% of their capacity, showcasing world-class performance. Moreover, the research demonstrated that the protective layer decomposes completely within about one month in soil, confirming its eco-friendly properties throughout its lifecycle—from production to disposal (Figure 2B).
Professor Il-Doo Kim stated, "By combining physical and chemical protective functions, we were able to more effectively facilitate reversible reactions between lithium metal and electrolytes while suppressing dendritic lithium growth. This enabled us to develop lithium-metal anodes with groundbreaking lifespan characteristics." He further emphasized, "With the growing demand for batteries, the environmental burden from their production and disposal has become a pressing issue. This water-based, eco-friendly manufacturing method and the naturally biodegradable properties of our protective layer will make a significant contribution to the commercialization of next-generation green batteries.”
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more