KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Fall 2025 Vol. 25A Better membrane paves the way for broader access to clean water
The development of a chlorine-resistant reverse osmosis membrane has been the industry's holy grail for the past decades. In this paper, a novel polyester TFC-RO membrane with remarkable water permeability, high rejection for salt and boron, and complete resistance toward chlorine was developed.
The global challenge of water scarcity is more pressing than ever, making desalination a vital part of the solution. However, current desalination technologies for removing salt from seawater are expensive and require frequent maintenance.
A research team has now created a robust and cost-effective filtration membrane that could significantly expand access to clean, potable water worldwide. This groundbreaking work, led by Prof. Menachem Elimelech from Yale University and Prof. Xuan Zhang from Nanjing University of Science & Technology, has been published in Science. Prof. Chanhee Boo and his team at KAIST made a significant contribution by conducting an in-depth evaluation and analysis of the membrane's essential properties and performance.
Reverse osmosis, a widely used water purification process that forces water through an ultra-fine semi-permeable membrane, has proven effective in delivering safe drinking water to regions in need. The dominant polyamide membranes used in this process provide excellent water permeability and salt rejection but are prone to biofouling, where bacterial films clog the membrane. Chlorine can mitigate biofouling but often damages the polyamide membranes. To address these challenges, industries rely on costly pretreatment methods.
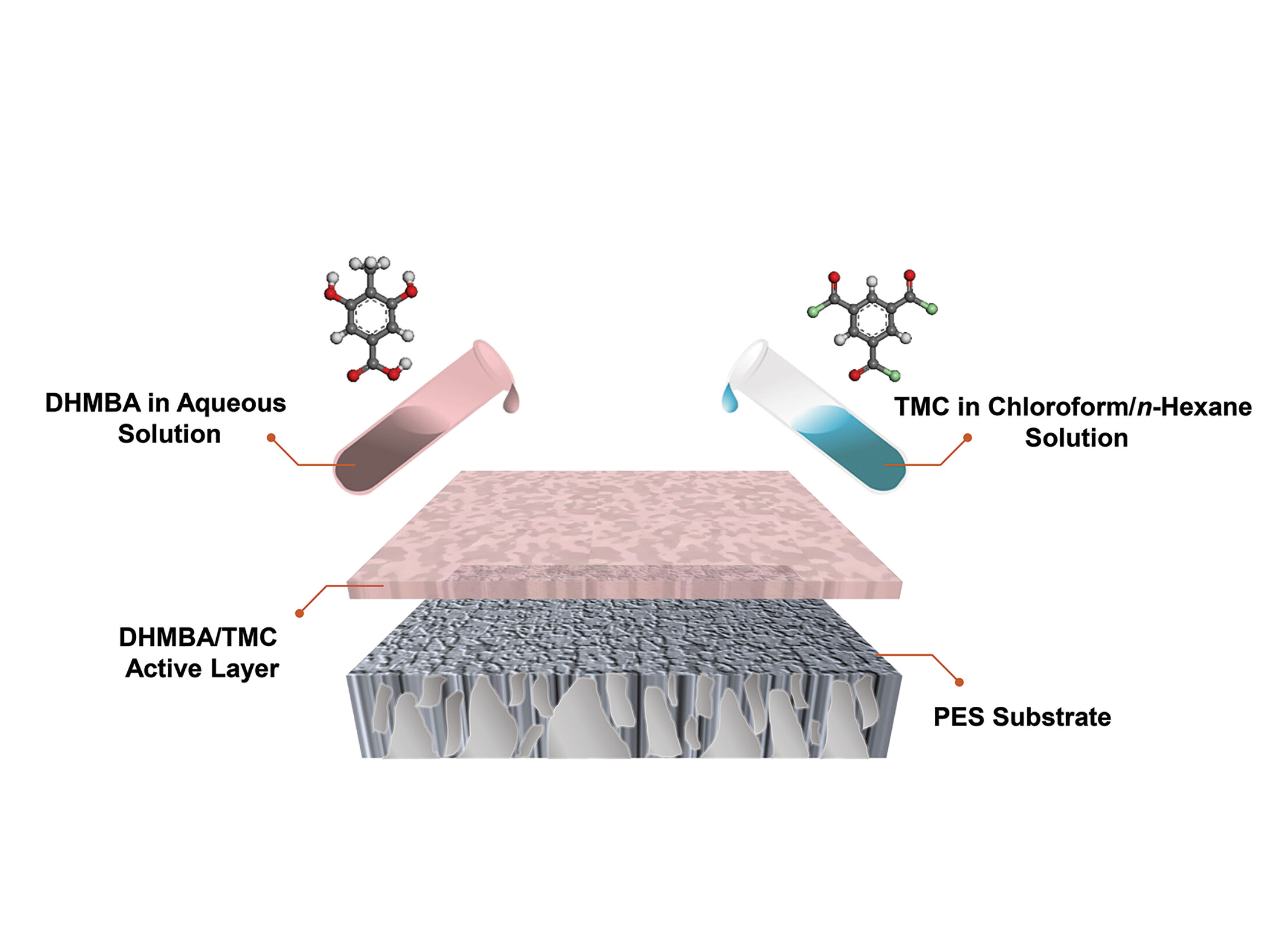
The researchers from Yale, Nanjing, and KAIST have now designed an innovative reverse osmosis membrane that resists both chlorine degradation and biofouling while effectively desalinating water (Figure 1). Instead of the conventional polyamide, they employed polyester to construct these membranes.
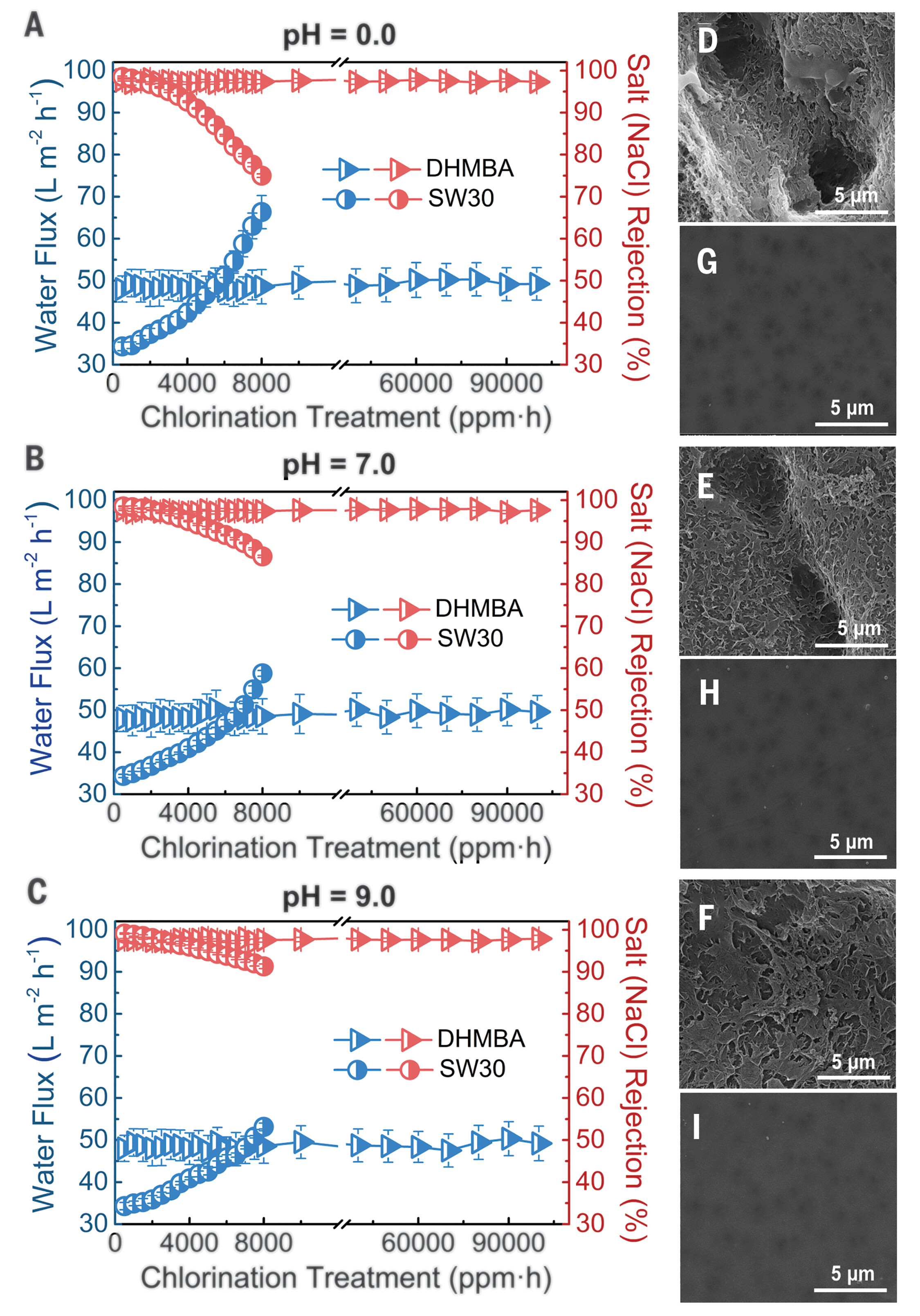
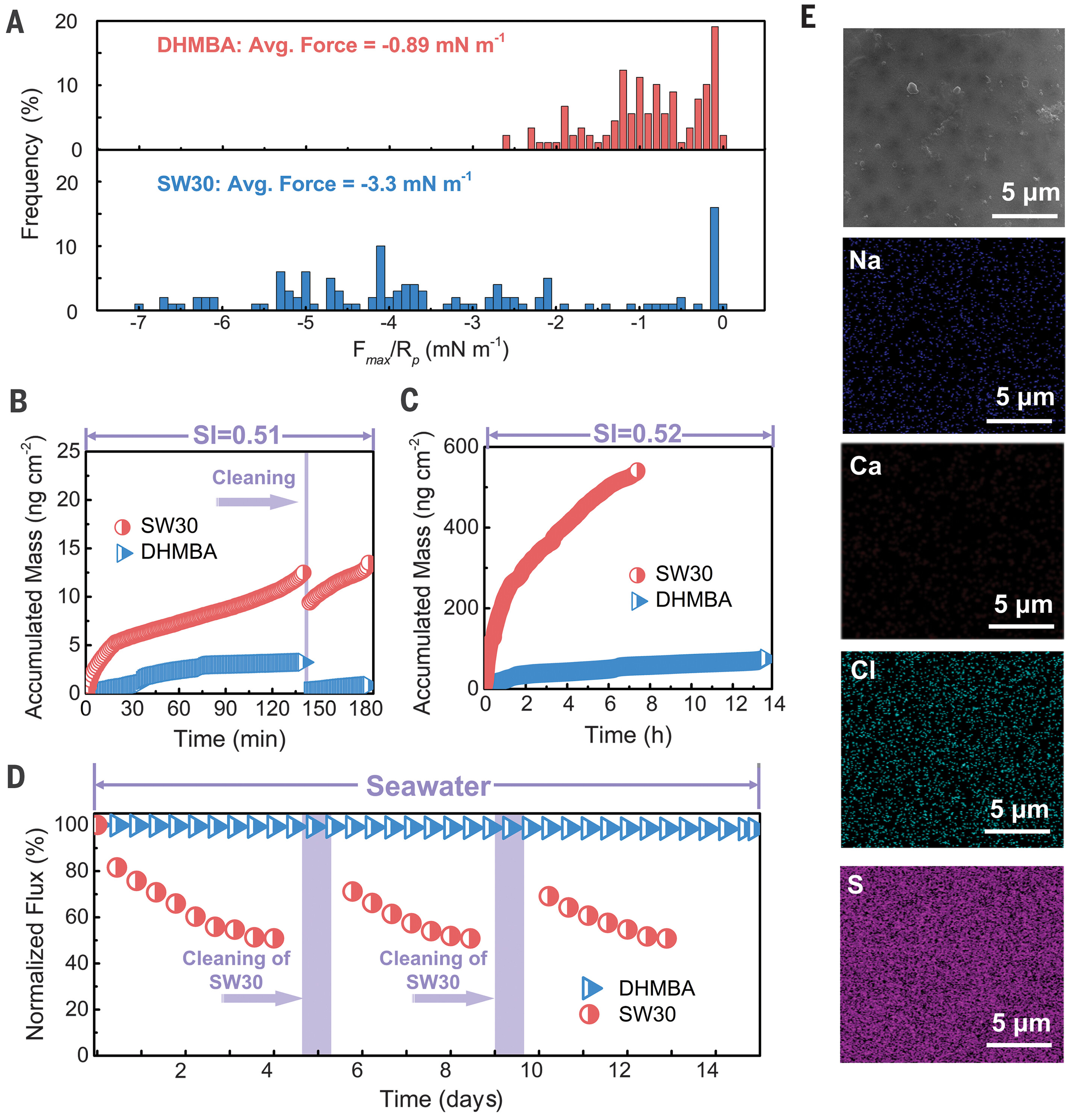
The choice of polyester is transformative: this material not only offers high water permeability and efficient rejection of sodium chloride and boron but is also completely resistant to chlorine. In addition, the membrane's ultra-smooth, low-energy surface minimizes fouling and scaling, outperforming traditional polyamide membranes (Figure 2).
Furthermore, the team ensured the new membranes are industry-ready. Their fabrication process closely aligns with existing production methods for polyamide membranes, allowing for seamless integration into current manufacturing systems and rapid scalability.
Most Popular

Soft Airless Wheel for A Lunar Exploration Rover Inspired by Origami and Da Vinci Bridge Principles
Read more
Wearable Haptics of Orthotropic Actuation for 3D Spatial Perception in Low-visibility Environment
Read more
Lighting the Lunar Night: KAIST Develops First Electrostatic Power Generator for the Moon
Read more
How AI Thinks: Understanding Visual Concept Formations in Deep Learning Models
Read more
TwinSpin: A Novel VR Controller Enabling In-Hand Rotation
Read more