KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24Highly Active and Durable Fuel Cell Catalysts Based on Block Copolymer
Highly Active and Durable Fuel Cell Catalysts Based on Block Copolymer
The proton exchange membrane fuel cell (PEMFC) has been used as the main power source of hydrogen cars. Here, the PEMFC catalyst has been developed using block copolymer-based carbon and an ultralow Pt loading. The PEMFC showed high power density and exceptionally high durability in spite of the ultralow Pt usage.
Article | Special Issue
Proton exchange membrane fuel cells (PEMFCs) are considered as future power sources, e.g., for hydrogen cars, because of high energy efficiency and zero pollutant emission. However, the market expansion of PEMFCs has been hindered by high cost and scarcity of Pt catalysts.
A research team of Prof. Hyunjoo Lee and Prof. Bumjoon J. Kim in Department of Chemical and Biomolecular Engineering at the Korea Advanced Institute of Science and Technology (KAIST) successfully developed the PEMFC catalyst in which the Pt catalyst formed an alloy with Fe and encapsulated with carbon shells. Although the Pt content was much lower (1 wt%) than commercially available Pt/C catalysts (>20 wt%), the new catalyst showed even higher power density and durability than the commercial Pt/C catalyst.
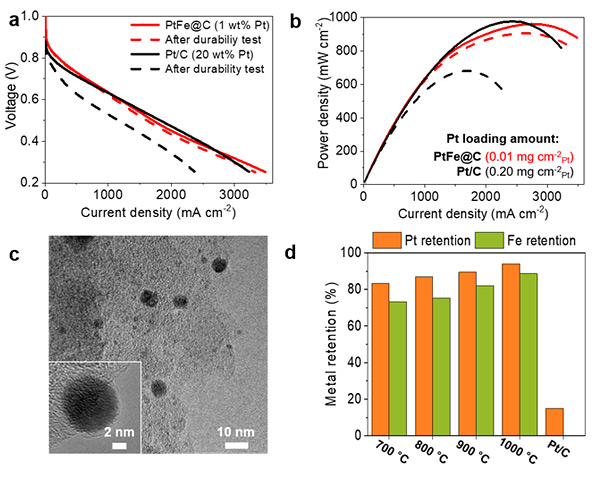
Prof. Bumjoon J. Kim’s group synthesized block copolymer-based spherical carbon supports with ~25 nm pores (Figures 1a and b) and Prof. Hyunjoo Lee’s group synthesized PtFe nanoparticles on the carbon support (Figures 1c and d). Interestingly, thin carbon shells were formed on the PtFe nanoparticles (PtFe@C). When the PtFe@C containing 1 wt% Pt was used as a cathode catalyst for oxygen reduction reaction, the power density was as high as commercial Pt/C containing 20 wt% Pt (Figures 2a and b).
More importantly, the power density of the PtFe@C was barely changed after a durability test, which was performed by repeating cyclic voltammetry in 0.6 V ~ 1.0 V for 30,000 cycles, whereas the power density of the commercial Pt/C was degraded significantly. This exceptionally high durability was due to the carbon shell on the metal nanoparticles. When the metal nanoparticles were exposed in highly acidic solution, the Pt was easily dissolved and no nanoparticles were left. However, the PtFe nanoparticles survived because the carbon shell protected the underlying nanoparticles (Figures 2c and d).
This new catalyst will provide a new platform to design an efficient PEMFC catalyst with minimum Pt use. This research was published in Energy & Environmental Science under the title of “Highly Durable Fuel Cell Catalysts Using Crosslinkable Block Copolymer-Based Carbon Supports with Ultralow Pt Loadings” (DOI: 10.1039/D0EE01095B).
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more