KAIST
BREAKTHROUGHS
Research Webzine of the KAIST College of Engineering since 2014
Spring 2025 Vol. 24A Novel Approach to Reduce Usage of Precious Catalysts for Hydrogen Production
A Novel Approach to Reduce Usage of Precious Catalysts for Hydrogen Production
Water electrolysis can efficiently store surplus electricity in the form of hydrogen, a clean future fuel. However, use of precious catalysts limits the dissemination of method of energy storage. This study presents TiO2-MoOx oxide as a support for iridium (Ir) nanoparticle catalyst. By introducing the TiO2-MoOx support to the water electrolysis, use of Ir can be reduced by 50% without performance loss owing to the fine dispersion and strong metal-support interaction.
Article | Special Issue
With the increase in global attention to climate change, the capacity of renewable power sources installed worldwide has increased. For efficient utilization of intermittent renewable energy, surplus electricity needs to be properly stored. As an energy storage technology, polymer electrolyte membrane water electrolysis (PEMWE) has attracted a great deal of attention owing to its ability to operate at a high current density over a wide load range, to respond rapidly to load changes, and to produce high purity hydrogen.
However, the commercialization of PEMWE is hindered due to the high fabrication cost and low durability, mostly related to the strongly-corrosive environment of the anode. In addition to the harsh conditions, the sluggish oxygen evolution reaction (OER) limits anode materials to precious iridium(Ir)-based catalysts, whose catalytic activity and durability should be improved for implementation of PEMWE. To achieve the goal, nanostructured Ir-based catalysts have been extensively studied. Although those catalysts have exhibited better OER activity than that of Ir, cost and durability issues have not yet been resolved.
Prof. Eun Ae Cho’s group in the Department of Material Science and Engineering, KAIST proposed a novel strategy to improve the activity and durability of Ir nanoparticles: the introduction of supports. With support, nanoparticles can be finely dispersed and prevented from agglomeration. The research group has presented a titanium oxide and molybdenum oxide composite (TiO2-MoOx) as a support for the Ir catalyst (Ir/TiO2-MoOx). Incorporation of higher valance cations, Mo(V) and Mo(VI), into TiO2 remarkably enhanced electrical conductivity.
Ir/TiO2-MoOx shows OER activity and durability superior to those of Ir black, Ir/C, and Ir/TiO2. As a result of the strong interaction between the oxide and Ir, Ir/TiO2-MoOx is found to promote the formation of OER-active Ir(III) species and to suppress further oxidation of Ir(III) to Ir(VI), even during OER. With increasing applied potential, oxidation of Ir species is most effectively suppressed and a higher fraction of OER-active Ir(III) on TiO2-MoOx is maintained. Ir/TiO2-MoOx achieves superior activity and durability through Mo-Ir interaction, forming, and retaining Ir(III) during OER. The single cell test results demonstrate that, as an anode catalyst, Ir/TiO2-MoOx can improve the performance and durability of PEMWE than the conventional Ir black. This research was published in Applied Catalysis B: Environmental under the title of “Stabilizing role of Mo in TiO2-MoOx supported Ir catalyst toward oxygen evolution reaction” (DOI:10.1016/j.apcatb.2020.119433).
Most Popular

When and why do graph neural networks become powerful?
Read more
Smart Warnings: LLM-enabled personalized driver assistance
Read more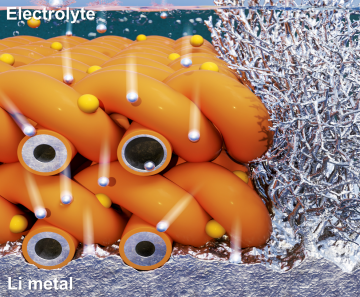
Extending the lifespan of next-generation lithium metal batteries with water
Read more
Professor Ki-Uk Kyung’s research team develops soft shape-morphing actuator capable of rapid 3D transformations
Read more
Oxynizer: Non-electric oxygen generator for developing countries
Read more


